Poly(ε-caprolactone)-Polypeptide Copolymer Micelles Enhance the Antibacterial Activities of Antibiotics
作者:Chen, L. S.; Fan, Z*; Du, J. Z.* 时间:2019-12-20 点击数:
Abstract:
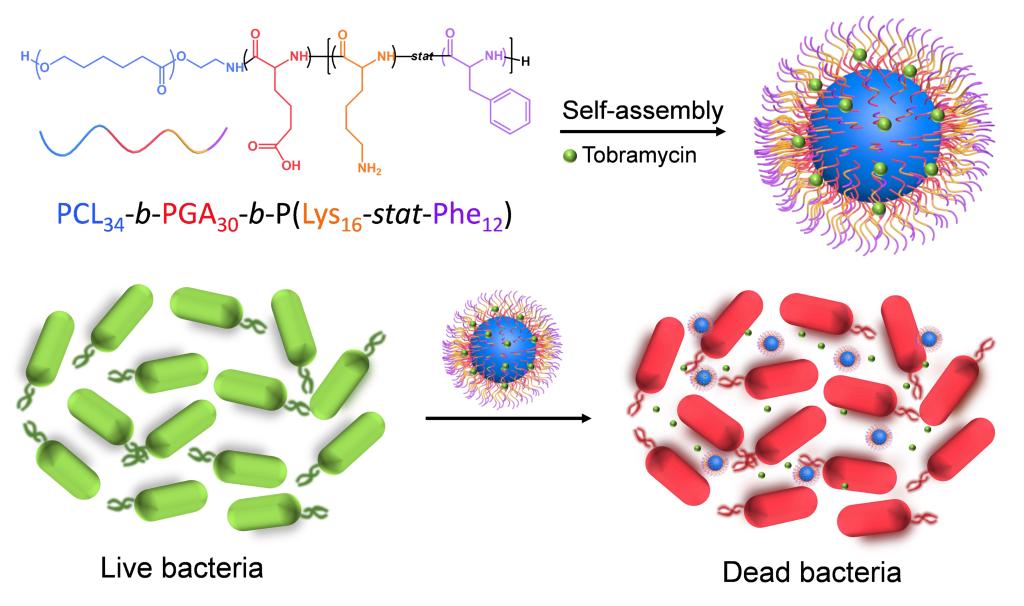
Bacterial infection is a major threat to human health, and can cause several diseases including gastroenteritis, influenza, tetanus, and tuberculosis. As conventional antibiotic treatment may cause various undesirable effects such as stomach disorder and bacterial resistance, it is necessary to improve the antibacterial efficiency of antibiotics. Here, we synthesized a peptide-based copolymer, poly(ε-caprolactone)-block-poly(glutamic acid)-block-poly(lysine-stat-phenylalanine)[PCL34-b-PGA30-b-P(Lys16-stat-Phe12)] by ring-opening polymerization (ROP) of ε-caprolactone and amino acid N-carboxyanhydride (NCA). Successful synthesis of the copolymer was verified by proton nuclear magnetic resonance and size exclusion chromatography. This copolymer can self-assemble into negatively charged micelles (-26.7 mV) under alkaline conditions by solvent switch method. The micelle structure was confirmed by transmission electron microscopy and dynamic light scattering, and revealed to have a diameter of~42 nm. Antibiotics were loaded into micelles during the self-assembly process, and cell viability assay was conducted to evaluate its cytotoxicity with and without tobramycin. No obvious cytotoxicity was observed for both micelles when the concentration was lower than 300 μg·mL-1. The antibiotic-loaded micelles demonstrated very low minimum inhibitory concentrations (MICs) against both Gram-negative Escherichia coli (E. coli) (7.8 μg·mL-1) and Gram-positive Staphylococcus aureus (S. aureus) (18.2 μg·mL-1), while the MICs of free tobramycin were 3.9 and 1.0 μg·mL-1, respectively. The drug-loading content and efficiency of the micelles were 5.2% and 24.3%, respectively. Therefore, the MICs of the loaded tobramycin against E. coli and S. aureus were 0.4 and 0.9 μg·mL-1, respectively, suggesting that the micelle could enhance the antibacterial activity of antibiotics. Tobramycin-loaded micelles demonstrated a sustained release characteristic, with 85% of the antibiotics released after 8 h. In bacteria-induced acidic microenvironment, the coil conformation of PGA blocks transforms and PGA blocks shrink toward the micelle core. Concomitantly, the carboxyl side chains are protonated in an acidic environment, increasing the hydrophobicity of this micelle. Antibiotics will be captured when reaching the outer core to slow down the releasing process. Furthermore, the poly(lysine-stat-phenylalanine)[P(Lys-stat-Phe)] coronas with broad spectrum intrinsic antibacterial activity can penetrate the bacterial cell membrane, leading to leakage of the cellular contents of the bacteria and ultimately their death. Due to the sustained release property of micelle and the intrinsic activity of the antibacterial peptide segments, this micelle can greatly enhance the antibacterial activity of antibiotics. Overall, this antibiotic-loaded micelle provides a novel approach for significantly reducing the antibiotics dosage and avoiding the associated health risks.
文章链接:Acta Physico-Chimica Sinica 2020, 36, 1910059.