Abstract
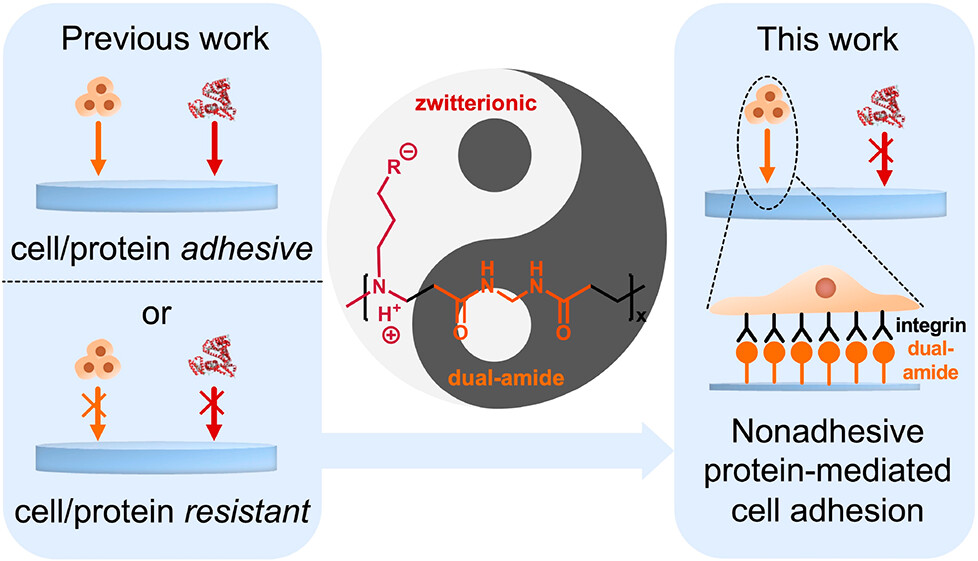
Cell adhesion plays a crucial role in determining the functionality of hydrogel-based culture platforms or implants and is commonly mediated by adhesive proteins. However, nonspecific protein adhesion is prone to causing inflammatory response in vivo. Traditional hydrogels are usually either pro- or anticell/protein adhesion simultaneously. To address this dilemma, a series of novel bioabsorbable poly(amidoamine) (PAA) hydrogels [aminobutyric acid hydrogel (C-Gel), aminopropanesulfonic acid hydrogel (S-Gel), and aminopropylphosphonic acid hydrogel (P-Gel)] are prepared, combining dual-amide (pro-cell adhesion) and zwitterionic (anti-protein adhesion) moieties, to simultaneously achieve excellent protein repulsion and cell adhesion. In our hydrogel system, the protein repulsion capability of hydrogels depends on the types of zwitterionic moieties (C-Gel > S-Gel > P-Gel) and the surface zwitterionic density. Meanwhile, the cell adhesion capability of hydrogels depends on the surface dual-amide density. C-Gel and S-Gel exhibit better cell adhesion compared to P-Gel due to their high surface dual-amide density. Additionally, the PAA hydrogels (especially C-Gel) have excellent cell proliferation and infiltration abilities for three-dimensional endothelial cell culture and thus achieving vascularization. Moreover, PAA hydrogels exhibit excellent degradation properties and biocompatibility in vitro and in vivo with no observed inflammatory responses. Therefore, these hydrogels hold substantial promise as platforms for tissue engineering, bringing new hope to promote the clinical translation of implantable hydrogels.
文章链接:Chemistry of Materials. 2023, 35, 21, 9208–9224