Abstract
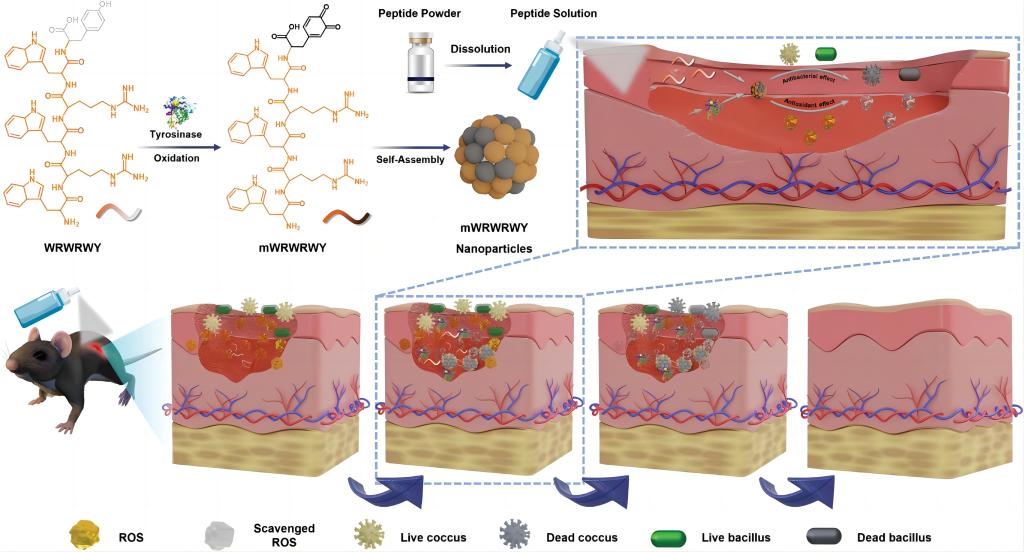
Antimicrobial peptides (AMPs) with dual intrinsic antibacterial and antioxidative functions have emerged as promising choice to cure infected wound. However, the most widely applied approach to endow AMPs with antioxidative function is to combine with antioxidative moieties, which may affect the spatial structure and physiological stability of AMPs. Herein, a new type of AMPs with inherent desired stability, antibacterial activity, and reactive oxygen species (ROS) scavenging is developed to effectively heal the infected wound. This formulation is in situ formed at wound site by tyrosinase-triggered oxidation and self-assembly of lyophilized antimicrobial peptide Trp-Arg-Trp-Arg-Trp-Tyr, providing enhanced stability and a fourfold and sevenfold increasement in antibacterial efficiency against E. coli and S. aureus compared to peptide monomers. The antimicrobial peptide is first oxidized and then assembled into nanoparticles. The melanin-like structure has been demonstrated with efficient antioxidant properties, and the experimental data show that peptide nanoparticles to scavenge superoxide radicals, hydroxyl radicals, and H2O2. In vivo experiments confirmed that peptide nanoparticles effectively heal infected wounds and obviously reduce ROS. Overall, the research provides a new approach to formulating antimicrobial peptides to treat wound with high healing efficiency.
文章链接:Adv. Funct. Mater. 2023, 2214454