Abstract
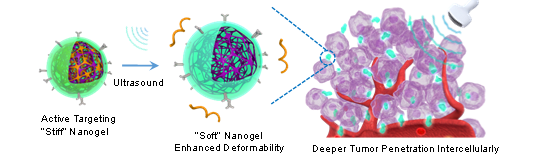
A series of biological barriers need to be overcome for therapeutic nanocarriers accumulating at the tumor site and uptaken by cancer cells. One strategy is to construct switchable nanocarriers to meet the conflicting requirements for various physiology environments. In this work, besides widely studied endogenous stimuli-responsiveness, an exogenous ultrasound responsiveness was additionally embedded into nanocarriers to balance the conflicting needs of prolonged blood circulation and deep tumor penetration. Polylysine and Pluronic F127 were first coassembled and then cross-linked by genipin to form stable nanogel structure. Subsequently, ICAM-1 antibody was grafted onto the nanogel (designated as GenPLPFT) for active tumor targeting. Upon external sonication, the F127 was shed from GenPLPFT to induce swelling of nanogel with reduced stability and accelerated drug release. In detail, sonication leads to GenPLPF swelling from 329 to 516 nm, while its Young’s modulus significantly decreased from 336.78 to 3.93 kPa. Through intravenous injection, relatively rigid GenPLPFT was able to achieve a high level of accumulation at tumor site by active targeting and long-term blood circulation. Moreover, under sonication at the tumor site, GenPLPFT became softer with enhanced deformability to achieve deep tumor penetration. In addition, in vivo studies revealed that GenPLPFT was able to penetrate into the deep area of xenografted tumor with enhanced antitumor efficacy and reduced toxicity. Overall, this peptide nanogel with ultrasound-responsive stiffness demonstrates an effective approach to overcome a series of biological barriers for enhanced deep tumor therapy.
文章链接:ACS NANO 2022, 6, 9183-9194.